📌 What You Need to Know
- NDT is being reclassified — The FDA now considers Natural Desiccated Thyroid an unapproved biologic.
- 12-month deadline — Doctors have 12 months to transition patients to FDA-approved synthetic thyroid medications.
- To stay on the market — Companies must get full biologics license approval, which requires expensive, years-long trials.
- Most brands may vanish — Smaller companies likely can’t meet the requirements, leaving only big pharma contenders.
- One major player — AbbVie, maker of Armour Thyroid (and synthetic T4), is running a massive trial that could secure approval – but could also lead to a monopoly and higher prices.
- Important note — The FDA’s faced pushback before when removing certain supplements off the market, and in some cases, enforcement timelines have been extended.
- Read the FDA’s official statements here: FDA’s Actions to Address Unapproved Thyroid Medications.
📌 Important Updates
- August 13th, 2025 — Just days after the FDA’s enforcement letters went out, Dr. Marty Makary (Chief of Clinical Policy at the FDA) posted this:
FDA’s Latest Move Could Remove Natural Desiccated Thyroid from the Market
FDA’s Enforcement Begins
If you take Armour, NP Thyroid, or any other form of natural desiccated thyroid (NDT), the FDA just made an announcement that could change your prescription – and possibly impact your health – within the next year.
On August 6, 2025, The FDA sent letters to manufacturers, informing them that NDT, a medication many patients rely on, is now being classified as an unapproved biologic.
A biologic is a medication made from living sources – such as animal glands, blood components, or cells. And, the FDA requires special licensing and clinical trials before it can be manufactured or sold.
12-Months to Get Patients onto Synthetic Thyroid Medications
The FDA has given doctors a 12 month transition period where they need to get their patients off of the natural thyroid medication and onto a synthetic FDA-approved medication instead.
To keep NDT on the market after that deadline, companies will need an approved Biologics License Application (BLA) – which requires extensive clinical trials, safety and efficacy data, and formal FDA review. This process takes a long time, and a lot of money.

What is Natural Desiccated Thyroid (NDT)?
Natural desiccated thyroid, or NDT, is a prescription medication made from the dried thyroid glands of pigs. It’s been used since the 1800s – long before the modern FDA approval process existed – and contains a mix of all the main thyroid hormones your body would normally produce.
When the FDA began requiring proof of safety and efficacy for drugs in 1938, NDT was already in common use and was “grandfathered” in, meaning it could remain on the market without going through the modern approval process, as long as the formulation and conditions for use stayed the same.
The Hormones in NDT
- T4 – the storage form of thyroid hormone
- T3 – the active form that powers metabolism
- T2 and T1 – lesser-known thyroid hormones that may have roles in metabolism and overall health
- Calcitonin – a hormone involved in calcium regulation and bone health
This full spectrum of hormones is unique to NDT and not found in standard synthetic options.
How NDT Differs from Synthetic Thyroid Medications
Synthetic T4-Only (Levothyroxine)
Most doctors prescribe synthetic T4 (levothyroxine) alone. T4 must be converted by the body into T3 before it can be used. For some people, this process works fine. But, a significant percentage of patients have trouble converting T4 into enough T3 to feel well.
Synthetic T4/T3 Combination Therapy
Some patients do better on a combination of synthetic T4 and synthetic T3. But, it can be difficult because not all doctors are willing to prescribe T3. The patient will also need to have two prescriptions instead of one, which could cost more.
Why Patients Choose NDT
NDT provides both T4 and T3 in a single prescription, along with the other thyroid hormones the body naturally makes. Many patients report feeling better on this, compared to T4-only therapy. Some people also prefer a natural product over synthetic.
Thyroid Medication Challenges
Individual Responses Vary
One person may thrive on a certain brand, while another experiences side effects or poor symptom control with the same medication. Even switching between brands with the same active ingredient can cause changes in how a patient feels. While some patients do well on synthetic thyroid medications, especially when taking both T4 and T3, others do not. Some people report symptom-relief only when taking an NDT.
The Filler Ingredient Problem
Some thyroid medications contain low-quality fillers, dyes, or binding agents that can trigger reactions or interfere with absorption. For sensitive patients, these additives can make an otherwise effective medication unusable. Some people have allergies to lactose. And, even NDT products contain filler or inactive ingredients which can cause problems for some patients.
High-Quality Options Come at a Price
Cleaner formulations do exist. Tirosint is a brand of T4 that’s free from many common fillers and contains minimal, clean ingredients. But, it’s far more expensive and often not covered by insurance. For many patients, cost becomes a major barrier to getting the medication that works best for them.
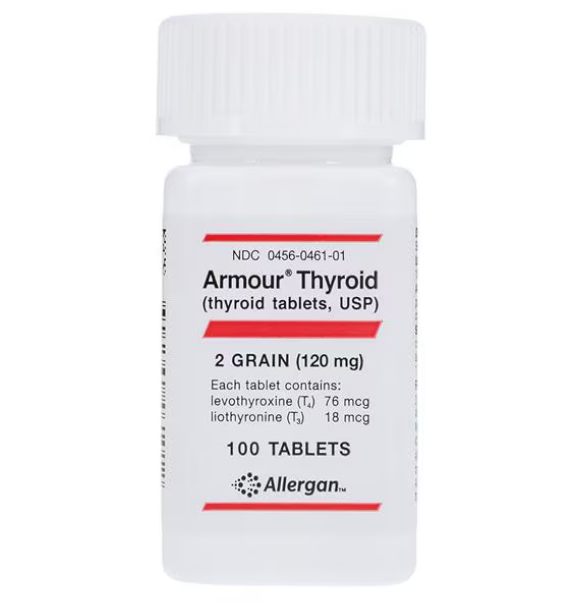
Who Makes NDT in the U.S.?
Several companies have produced NDT over the years:
- Armour Thyroid – made by AbbVie, currently the most widely available brand
- NP Thyroid – made by Acella Pharmaceuticals
- Nature-Throid – made by RLC Labs (discontinued in recent years)
- Compounded NDT – made by specialty pharmacies in customized doses and formulations
What to Expect: Will NDT Disappear for Good?
There’s no way of knowing for sure what will happen. Based on the latest FDA letter, patients are supposed to transition off of NDT and onto a synthetic thyroid medication over the next 12 months. The requirements necessary for a biologics license would take years, and millions of dollars. Many small pharmaceutical companies will not have the funding or resources to meet these requirements.
Right now, based on my research, it appears there’s one main company with the resources, infrastructure, and regulatory experience to meet the FDA’s new standards – though not within the short timeline.
What No One Is Talking About
I discovered a major clinical trial that’s recruiting right now, and it could be the key to bringing NDT back under the FDA’s new rules.
The Clinical Trial that Could Save Armour NDT – But at What Cost?
Right now there is one company, AbbVie, that appears to be actively taking steps toward meeting the FDA’s biologics requirements. AbbVie isn’t just another drugmaker – they’re one of the largest pharmaceutical companies in the world. And, they are the maker of Armour, the most popular NDT on the market. They also sell synthetic T4 (levothyroxine), which is the most common synthetic thyroid medicine prescribed in the U.S.
During my research, I uncovered something that hasn’t been widely discussed and, as far as I can tell, hasn’t been reported on anywhere else: AbbVie is currently recruiting for a massive, multi-state clinical trial comparing Armour Thyroid to synthetic T4. (See clinical trial details here)
Armour Thyroid NDT T4 Trial Details
This double-blinded, randomized study is enrolling roughly 2,800 participants and will measure both safety and effectiveness, along with how patients respond when switching from T4 to Armour. The trial is scheduled to run through 2027 and conclude in 2028. It appears this could be part of AbbVie’s effort to secure the Biologics License required to keep NDT legally on the market. If you’re interested in being a part of the study, see study and recruitment details on their website here.
Potential Monopoly on the Thyroid Medication Market
If AbbVie succeeds, and if no other manufacturers meet the biologics requirements, then they could end up with a monopoly on NDT in the United States. Smaller companies may not have the resources for multi-year, multi-million-dollar trials or the specialized legal and regulatory teams needed to handle the biologics approval process.
And when there’s only one supplier, the prices could go up. This is a big problem because Armour is already a very expensive medication and for some patients, it’s not covered by insurance.
What About the Other Brands?
I’ll be reaching out to other NDT manufacturers to see if I can get more information on what their plans are. If they respond, I’ll share those updates in future videos and blog posts so patients can stay informed.

History of the FDA NDT Issues
FDA Issues Started in 2009, Escalated in 2022 & Now in 2025
This fight isn’t entirely new. Some patient advocacy groups say the FDA first made moves against NDT back in 2009, raising questions about its manufacturing standards and hinting at tighter restrictions. While I haven’t been able to find an official FDA statement from that time, it’s clear that concerns about NDT have been simmering for well over a decade.
Then in 2022, the agency quietly sent a letter to the National Association of Boards of Pharmacy (NABP) saying that NDT should be considered a biologic — and therefore ineligible for compounding without a biologics license.
Compounding Pharmacies and NDT
Compounding pharmacies make customized medications for patients. For thyroid patients, compounding is often the only way to get a specific NDT dose or formulation that works for them, because thyroid issues are complex and each patient’s dose and medication requirements are different.
Although that 2022 letter wasn’t a public announcement, it was a clear signal to state pharmacy boards to start restricting compounding.
At the time, some believed companies would have until 2029 to get biologics approval before any major enforcement would happen.
But that’s now changed — as the FDA’s current deadline is in 12 months.
Could the Timeline Change?
It’s worth noting that the FDA has a history of adjusting or delaying enforcement when public pushback is strong enough. For example, some supplements they wanted to ban remained available for years after the initial announcement. While there’s no guarantee the same will happen with NDT, it’s a possibility worth keeping in mind.





